Overview
We recognize that the essential features of clinical trial platforms are pivotal for success. Robust data security, real-time monitoring, efficient data management, and user-friendly interfaces are not just beneficial; they are crucial for maintaining data integrity and ensuring regulatory compliance. These features streamline operations and enhance collaboration among our research teams, significantly improving patient outcomes and ensuring adherence to regulatory standards. This, in turn, drives the overall success of clinical trials.
What challenges are holding your team back? By integrating these vital elements, we can transform your clinical trial processes into a model of efficiency and effectiveness. Let’s work together to achieve excellence in our clinical endeavors.
Introduction
In the realm of clinical trials, where every second counts and data integrity is paramount, we recognize that the need for a robust integration platform has never been more critical. Our hybrid integration platform, Avato, stands out as a beacon of reliability, ensuring 24/7 uptime and empowering clinical teams to focus on what truly matters: advancing medical research.
With advanced security measures, seamless integration of diverse data sources, and real-time monitoring capabilities, we transform the landscape of clinical trials. This article delves into the multifaceted advantages of our platform, exploring how it enhances operational efficiency, fosters collaboration, and ensures compliance, ultimately driving better patient outcomes in an ever-evolving healthcare environment.
What’s holding your team back from achieving these advancements? Let us guide you towards a solution that not only meets your needs but also elevates your research initiatives.
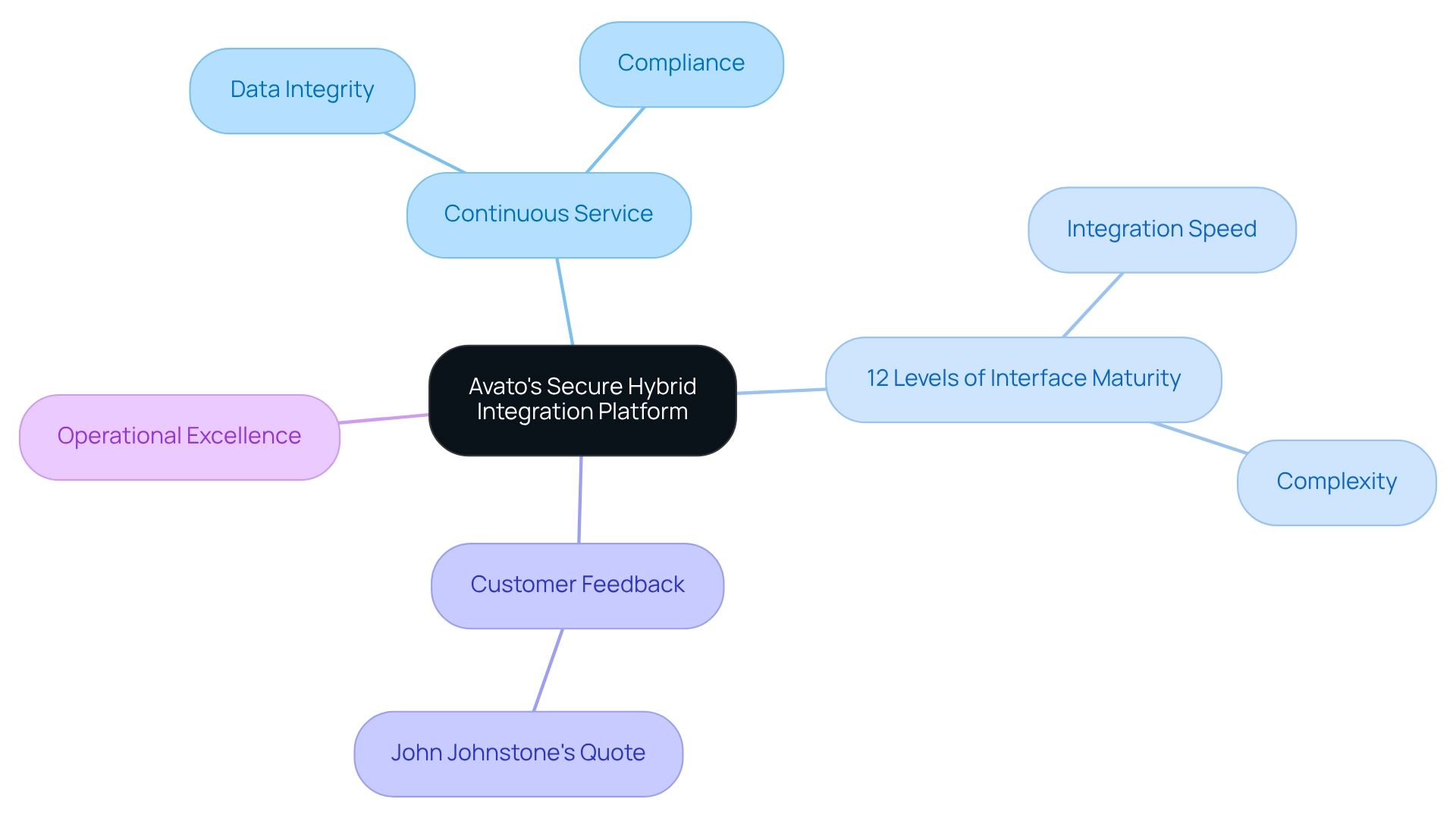
Avato’s Secure Hybrid Integration Platform: Ensuring 24/7 Uptime for Clinical Trials
We recognize that continuous service is not just a feature; it is a necessity for seamless research operations where data integrity and timely results are paramount. Our hybrid integration platform is meticulously crafted to support integration projects requiring 24/7 availability, ensuring that research teams can concentrate on their studies without the looming threat of system failures or disruptions. Did you know that average downtime in research trials can soar to 30%? Such interruptions can severely impede progress, highlighting the critical need for reliable clinical trial platforms.
Our solution accommodates 12 levels of interface maturity, allowing organizations to harmonize integration speed with the complexity needed to future-proof their technology stack. Industry leaders emphasize that continuous service on clinical trial platforms is vital for safeguarding the integrity of medical data and ensuring compliance with regulatory standards. As John Johnstone, one of our valued customers, stated, “The platform simplifies intricate projects and provides outcomes within preferred time limits and budget restrictions,” highlighting its efficiency in research environments.
Moreover, our unwavering commitment to minimizing downtime not only bolsters operational excellence but also fosters an environment where research can thrive, addressing the ethical, legal, and social challenges linked to IT compliance. We invite you to join us in harnessing this powerful solution to elevate your research capabilities.
Robust Data Security Measures: Protecting Sensitive Clinical Trial Information
At Avato, we recognize that safeguarding sensitive clinical trial information is paramount. We implement advanced security measures, including robust encryption protocols, stringent access controls, and comprehensive regular audits. These protocols are designed to ensure that only authorized individuals can access essential information, significantly reducing the risk of unauthorized entry and potential information breaches.
As we look ahead to 2025, organizations must record all breaches under GDPR to demonstrate adherence to protection principles. This underscores the importance of upholding regulatory standards. By prioritizing information security, we not only help organizations comply with these regulations but also build trust with study participants. What’s holding your team back from achieving the highest standards of data protection?
Cybersecurity specialists emphasize that effective information protection strategies are critical in medical studies, where the integrity of sensitive details is vital. As the saying goes, ‘an ounce of prevention is worth a pound of cure,’ highlighting the proactive approach necessary for protecting research information. Optimal methods involve utilizing encryption and access controls, which are essential for shielding research data from emerging threats.
Furthermore, with the recent updates to NIST SP 800-171A, we encourage organizations to stay informed about the latest guidelines, especially with a webinar scheduled for June 6, 2023. Our dedication to these measures positions us as a leader in ensuring the safety and confidentiality of research information. Case studies showcasing our successful implementations further validate our effectiveness in this critical area.
Integration of Diverse Data Sources: Enhancing Clinical Trial Insights
We excel in merging various information sources, such as electronic health records (EHRs), lab results, and patient-reported outcomes. This integration enables our trial teams to examine information from multiple perspectives, leading to greater insights and more efficient trial strategies. By breaking down barriers among different information sources, we significantly improve the quality of medical research.
For instance, Clinixir has successfully executed integration strategies utilizing Avato’s advanced capabilities, allowing pharmaceutical companies to streamline processes and accelerate the delivery of life-changing therapies. The incorporation of EHRs enhances patient histories and boosts study results, as unified datasets provide a comprehensive view of patient experiences and treatment responses, directly influencing research success.
As the landscape of medical studies evolves, the ability to leverage diverse information sources becomes increasingly essential. Industry leaders recognize that efficient information integration is crucial for achieving improved patient outcomes and advancing medical research.
With over 2,200 clients worldwide, we illustrate the growing significance of data integration in research studies, underscoring the relevance of Avato’s services in this dynamic environment. As Gustavo Estrada noted, ‘Avato has streamlined intricate projects and provided outcomes within preferred timelines and budget limitations,’ highlighting our efficiency in managing research challenges while ensuring secure transactions and operational excellence.
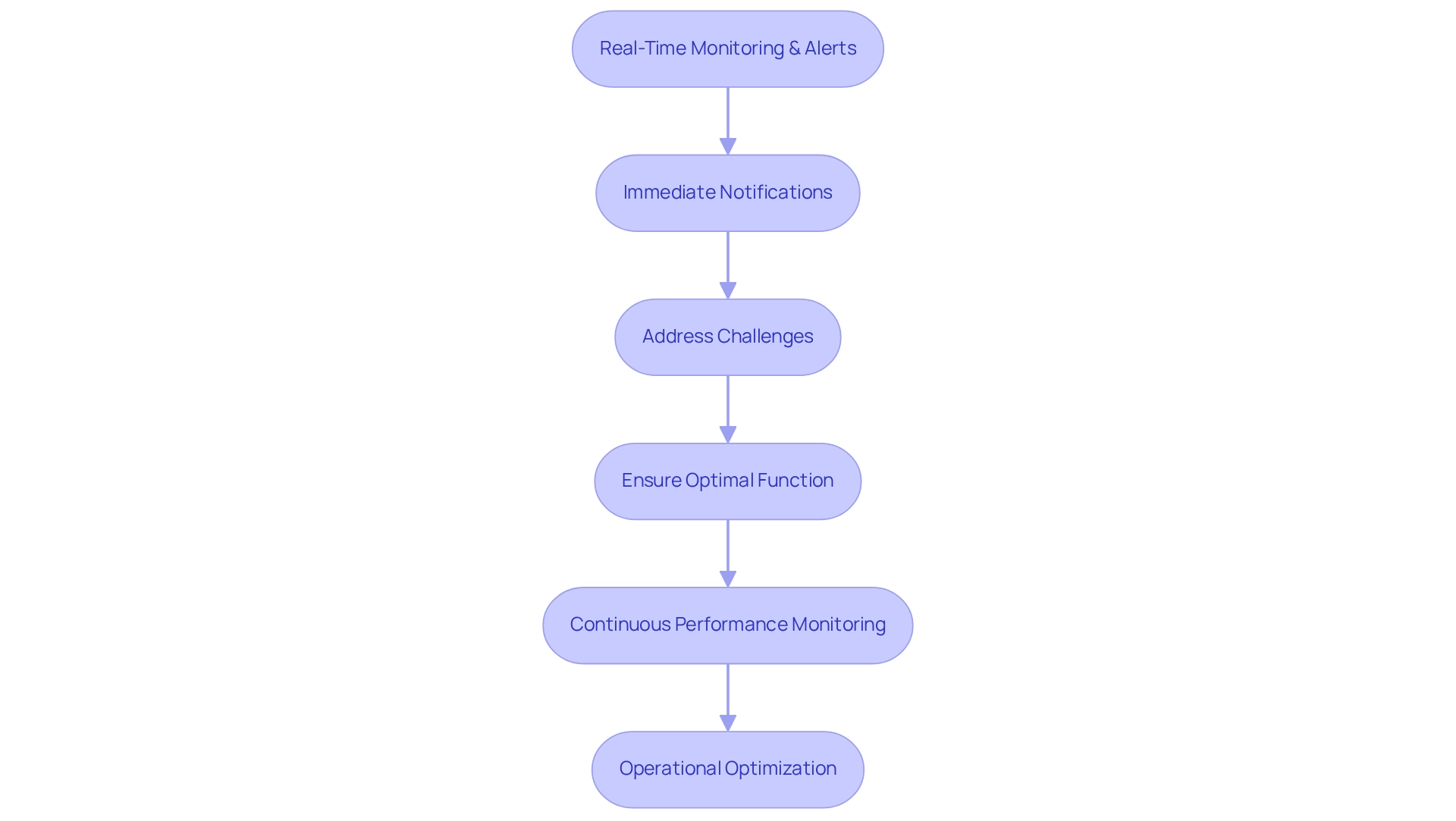
Real-Time Monitoring and Alerts: Maintaining Clinical Trial Integrity
Our real-time monitoring and alert system empowers research teams with immediate notifications concerning any irregularities or performance issues. This proactive strategy allows us to address potential challenges before they compromise results. By ensuring that all systems function optimally, we help preserve the integrity and reliability of medical studies.
Furthermore, our robust analytics capabilities on clinical trial platforms facilitate continuous performance monitoring, enabling ongoing operational optimization. This commitment to analytics not only enhances the flexibility of clinical trial platforms but also enables us to uncover new opportunities for innovation, ultimately driving the success of the trials.
What’s holding your team back from achieving these advancements? Let us partner with you to elevate your research efforts.
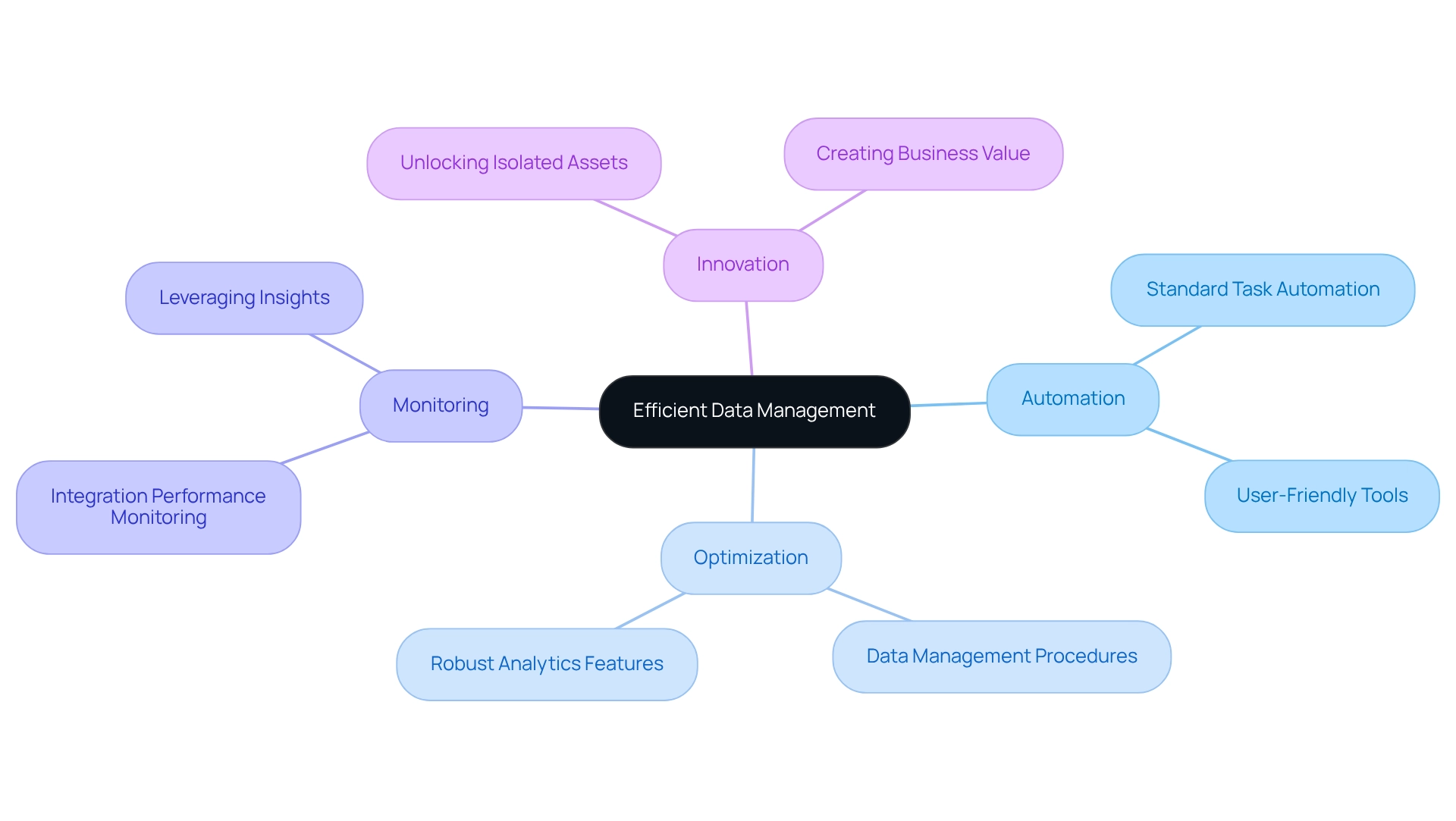
Efficient Data Management: Streamlining Clinical Trial Processes
We understand that managing information efficiently is a critical challenge in healthcare. Our hybrid integration platform simplifies this process by automating standard tasks and offering user-friendly tools for input and retrieval. This efficiency significantly reduces the time spent on administrative tasks, allowing our healthcare teams to focus on what truly matters: research and patient care. By optimizing data management procedures and providing robust analytics features, we enhance the overall efficiency of clinical trial platforms.
Furthermore, our ongoing monitoring and enhancement of integration performance ensure that healthcare teams can leverage insights to improve operations and uncover new opportunities for innovation. This ultimately leads to better patient outcomes. Additionally, our platform unlocks isolated assets, empowering organizations to create substantial business value and elevate their operational capabilities.
What’s holding your team back? We invite you to explore how our integration platform can transform your information management, driving efficiency and innovation in your organization.
Advanced Data Analytics: Driving Informed Decisions in Clinical Trials
At Avato, we harness sophisticated data analytics features that empower research teams to swiftly and precisely examine substantial amounts of data. By utilizing advanced machine learning algorithms and predictive analytics, we enable teams to identify trends, evaluate risks, and make informed choices that enhance outcomes. This analytical capability is essential for navigating the complexities of contemporary medical research.
Furthermore, our commitment to designing robust technological foundations facilitates the seamless integration of diverse systems, thereby empowering research teams to access isolated data resources and significantly enhance business value generation.
What’s holding your team back from achieving these advancements? Let us partner with you to unlock the full potential of your research capabilities.
Regulatory Compliance Features: Ensuring Adherence in Clinical Trials
At Avato, we recognize that effective regulatory compliance is paramount in research studies. Our clinical trial platforms are equipped with essential features such as robust audit trails, electronic signatures, and comprehensive documentation management, all designed to empower clinical trial teams. By maintaining precise records and adhering to stringent regulatory requirements, we ensure ethical and transparent trial conduct.
The significance of audit trails cannot be overstated; they serve as a safeguard against unauthorized manipulation, reinforcing the integrity of our processes. As Raja Rajeswari Kamisetti aptly states, “The audit trail shall be compared with the instrument log book on a regular basis,” underscoring the necessity for diligent oversight. A case study on audit trail management illustrates the critical need for implementing strict procedures to uphold compliance standards.
Moreover, our research indicates that pharmaceutical companies leveraging end-to-end data management solutions can enhance their chances of regulatory approval by 23%. By prioritizing adherence, we not only support the integrity of medical research but also enable organizations to navigate the complexities of regulatory environments effectively. As we look towards 2025, the growing emphasis on internal quality management systems suggests that enhancing these systems will lead to successful medical evaluations.
Our unwavering dedication to maintaining precise records and promoting compliance with regulations positions us as a frontrunner in clinical trial platforms. What’s holding your team back from achieving the same level of excellence? Let us partner with you to elevate your research capabilities and ensure compliance with confidence.
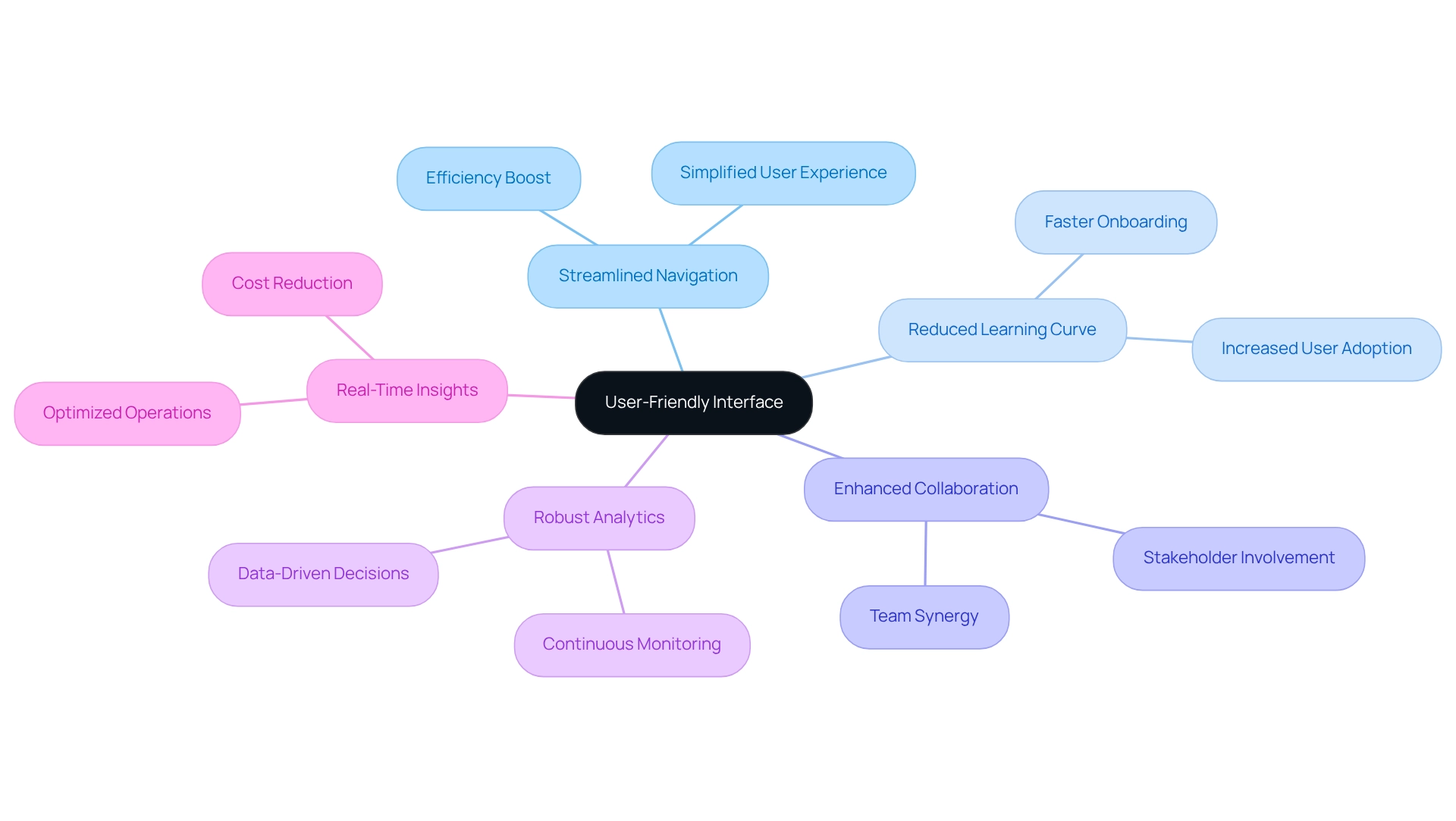
User-Friendly Interface: Enhancing Accessibility for Clinical Trial Teams
We offer clinical trial platforms that revolutionize clinical research. Imagine a solution that not only streamlines navigation and data input but also significantly reduces the learning curve for new users. This intuitive design fosters collaboration among our teams and enhances accessibility, enabling effective stakeholder involvement and ultimately boosting efficiency.
Furthermore, our robust analytics capabilities facilitate continuous monitoring of system performance, empowering us to optimize operations and enhance customer experiences. As Gustavo Estrada, Acting Provincial Director at BC Provincial Health Services Authority, noted, “The solution allowed us to align the outcomes we wanted to achieve in the time frame and price point we were targeting.”
This emphasis on real-time insights not only simplifies intricate integrations but also substantially lowers expenses, ensuring that our research studies on clinical trial platforms are conducted efficiently and effectively.
What’s holding your team back from achieving these results? Let us guide you toward a more streamlined and productive future.
Collaboration Tools: Facilitating Communication in Clinical Trials
We recognize that effective collaboration tools, such as clinical trial platforms, are essential for fostering seamless communication among clinical research stakeholders, including sponsors, investigators, and site personnel. By integrating features such as shared dashboards, messaging systems, and document sharing, we enhance teamwork and ensure alignment on project objectives. This cooperative approach not only streamlines testing procedures on clinical trial platforms but also significantly enhances outcomes.
A recent survey revealed that 83% of sponsors are prepared to share electronic Investigator Site Files (eISFs) with locations lacking them, emphasizing the growing need for efficient communication tools and clinical trial platforms in research studies. Furthermore, professional insights indicate that effective teamwork boosts revenue, profits, and employee satisfaction, highlighting the critical role of collaboration in achieving success in clinical trial platforms.
As Dr. Horst Stipp noted, ‘Statista offers the information you seek immediately,’ a necessity in the fast-paced realm of research studies. Additionally, a case study titled ‘Desire for Honest Communication in Organizations’ demonstrated that while 99.1% of employees wish to be part of organizations that promote honest communication, only 50% feel they are in such environments, illustrating the existing gap in communication practices.
Our commitment to enabling secure and compliant communication among stakeholders positions us as a leader in enhancing the management of research studies using clinical trial platforms.
Scalability Options: Adapting to Evolving Clinical Trial Needs
Our hybrid integration platform, aptly named for its dedication to excellence, offers robust scalability options that empower research teams to adjust resources and capabilities in alignment with study demands. Are you overseeing a small pilot study or a large-scale multi-site trial? We guarantee that your organization can expand operations efficiently by leveraging clinical trial platforms to address the evolving needs of clinical research while maintaining a competitive edge. By unlocking isolated assets, our platform delivers measurable results for our customers, reflecting our unwavering commitment to architecting technology solutions that enhance business value through seamless data and system integration. Let us partner with you to elevate your research capabilities.
Conclusion
As we navigate the complex landscape of clinical trials, Avato’s hybrid integration platform stands out as an indispensable asset, addressing the pressing demands for reliability, security, and operational efficiency. Our commitment to 24/7 uptime empowers clinical teams to concentrate on advancing medical research without the anxiety of system failures, thereby enhancing data integrity and ensuring compliance with regulatory standards. With robust security measures and advanced data analytics, we solidify our position as leaders in safeguarding sensitive information and facilitating informed decision-making.
The seamless integration of diverse data sources elevates the quality of clinical research, enabling teams to extract deeper insights and improve patient outcomes. By promoting real-time monitoring and efficient data management, we ensure that clinical trial operations remain agile and responsive to ever-evolving challenges. Our user-friendly interface and collaboration tools foster effective communication among stakeholders, cultivating a culture of teamwork and transparency that is essential for trial success.
As the complexity of clinical trials increases, our scalability options provide the necessary flexibility to adapt to changing demands. By unlocking isolated data assets and streamlining processes, the platform not only enhances operational capabilities but also drives significant business value. In a healthcare environment that is constantly evolving, embracing our comprehensive integration solutions is vital for organizations striving to excel in clinical research while upholding the highest standards of patient care and regulatory compliance. Let us partner with you to transform your clinical trial processes and achieve unparalleled success.

